Cavicularin
 | |
| Names | |
|---|---|
| IUPAC name 9,10,18,19-Tetrahydro-5,8:15,17-diethenobenzo[g]naphth[1,8-bc]oxacyclotetradecin-3,12,21-triol | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChemSpider |
|
PubChem CID |
|
| UNII |
|
CompTox Dashboard (EPA) |
|
InChI
| |
| |
| Properties | |
Chemical formula | C28H22O4 |
| Molar mass | 422.480 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).  N verify (what is N verify (what is  Y Y N ?) N ?) Infobox references | |
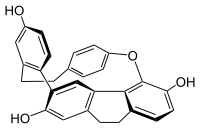
Cavicularin is a natural phenolic secondary metabolite isolated from the liverwort Cavicularia densa. This macrocycle is unusual because it was the first compound isolated from nature displaying optical activity solely due to the presence of planar chirality and axial chirality. The specific rotation for (+)-cavicularin is +168.2°.[1] It is also a very strained molecule. The para-substituted phenol ring is bent about 15° out of planarity, adopting a somewhat boat-like geometry. This type of angle strain in aromatic compounds is normally reserved for synthetic cyclophanes.

The liverwort was obtained from Mount Ishizuchi in the district of Shikoku. The material was dried for one day, ground to a powder and 5 grams were refluxed in methanol for 4 months [reference needed] to yield 2.5 mg (0.049%) of cavicularin after column chromatography and preparative TLC.
Total synthesis
In 2005[2] and again in 2011,[3] the compound was prepared by total synthesis together with the unstrained compound riccardin C. In 2013, several other syntheses were reported for it[4][5] and a racemic synthesis.[6]
References
- ^ M. Toyota; T. Yoshida; Y. Kan; S. Takaoka; Y. Asakawa (1996). "(+)-Cavicularin: A Novel Optically Active Cyclic Bibenzyl-Dihydrophenanthrene Derivative from the Liverwort Cavicularia densa Steph". Tetrahedron Letters. 37 (27): 4745–4748. doi:10.1016/0040-4039(96)00956-2.[dead link]
- ^ David C. Harrowven; Timothy Woodcock; Peter D. Howes (2005). "Total Synthesis of Cavicularin and Riccardin C: Addressing the Synthesis of an Arene That Adopts a Boat Configuration". Angewandte Chemie. 44 (25): 3899–3901. doi:10.1002/anie.200500466. PMID 15900530.
- ^ Kostiuk, S. L.; Woodcock, T.; Dudin, L. F.; Howes, P. D.; Harrowven, D. C. (2011). "Unified Syntheses of Cavicularin and Riccardin C: Addressing the Synthesis of an Arene Adopting a Boat Configuration". Chemistry: A European Journal. 17 (39): 10906–10915. doi:10.1002/chem.201101550. PMID 21932232.
- ^ Takiguchi, H.; Ohmori, K.; Suzuki, K. (2013). "Synthesis and Determination of the Absolute Configuration of Cavicularin by a Symmetrization/Asymmetrization Approach". Angew. Chem. Int. Ed. 52 (40): 10472–10476. doi:10.1002/anie.201304929. PMID 23956143.
- ^ Zhao, Peng; Beaudry, Christopher M. (2013). "Total Synthesis of (±)-Cavicularin: Control of Pyrone Diels–Alder Regiochemistry Using Isomeric Vinyl Sulfones". Organic Letters. 15 (2): 402–405. doi:10.1021/ol303390a. PMID 23301524.
- ^ Harada, Kenichi; Makino, Kosho; Shima, Naoki; Okuyama, Haruka; Esumi, Tomoyuki; Kubo, Miwa; Hioki, Hideaki; Asakawa, Yoshinori; Fukuyama, Yoshiyasu (2013). "Total synthesis of riccardin C and (±)-cavicularin via Pd-catalyzed Ar–Ar cross couplings". Tetrahedron. 69 (34): 6959–6968. doi:10.1016/j.tet.2013.06.064.
- v
- t
- e
- Batatasin-III (3,3′-dihydroxy-5-methoxybibenzyl)
- Combretastatin and combretastatin B-1
- Dihydro-resveratrol
- Isonotholaenic acid
- Lunularic acid
- Lunularin
- Notholaenic acid
- Tyrolobibenzyl A, B and C
- Bis(bibenzyls): 13,13'-O-Isoproylidenericcardin D
- Marchantin B and E
- Neomarchantin A
- Plagiochin E
- Riccardin H
- Macrocyclic bis(benzyls): Marchantin A and C
- Riccardin B and C
- Cyclic bibenzyl-dihydrophenanthrene derivative: Cavicularin











