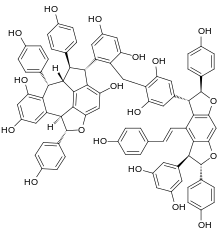
Chunganenol
 | |
| Names | |
|---|---|
| Preferred IUPAC name (21S,25R,26R,26aS,27S,211bS,62S,63S,65S,66S)-26,27,62,66-Tetrakis(4-hydroxyphenyl)-64-[(1E)-2-(4-hydroxyphenyl)ethen-1-yl]-21,25,26,26a,27,211b,62,63,65,66-decahydro-2(1,5)-benzo[3,4]azuleno[7,8,1-cde][1]benzofurana-6(3,5)-benzo[1,2-b:5,4-b′]difurana-1,7(1),3(1,2),5(1,4)-tetrabenzenaheptaphane-14,24,28,210,33,35,52,56,73,75-decol | |
| Identifiers | |
3D model (JSmol) |
|
PubChem CID |
|
CompTox Dashboard (EPA) |
|
InChI
| |
| |
| Properties | |
Chemical formula | C85H64O18 |
| Molar mass | 1373.429 g·mol−1 |
| Appearance | Colorless amorphous powder |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). Infobox references | |
Chemical compound
Chunganenol is a resveratrol hexamer found in Vitis chunganensis.[1]
References
- ^ Shan He; Liyan Jiang; Bin Wu; Chang Li; Yuanjiang Pan (2009). "Chunganenol: An Unusual Antioxidative Resveratrol Hexamer from Vitis chunganensis". J. Org. Chem. 74 (20): 7966–7969. doi:10.1021/jo901354p. PMID 19757796.
- v
- t
- e
Oligostilbenoids and their glycosides
- Diptoindonesin C
- Diptoindonesin F
- Gnetin H
- Hemsleyanol D
- Isohopeaphenol
- Laetevirenol A, B, C, D and E
- Suffruticosol A and B
- Viniferal
- E-ω-viniferin
- Z-ω-viniferin
- Diptoindonesin G
- Jezonodione
- B
- Scirpusin A
- Tibeticanol (piceatannol dimer)
- Amurensin B
- Gnetin E
- Gneyulin A
- Johorenol A
- Ampelopsin E
- Vaticanol G
- Dibalanocarpol
- Gnetin J (3"-hydroxygnetin E)
- Gnetin K (3"-methoxygnetin E)
- Gnetuhainin R (isorhapontigenin tetramer)
- Laetevirenol F and G
(five units or more)
- Vaticanol D, H, I and J
of resveratrol
| Dimers |
|
|---|---|
| Trimers |
|
| Tetramers |
|
| Pentamers |
|
| Hexamers |
|
| Higher polymers |
|
- Diptoindonesin A (C-glucoside of ε-viniferin)
- Foeniculoside I (glucoside of miyabenol C), II, III and IV
- Laevifonol (an ε-viniferin-ascorbic acid hybrid compound)
- Laevifoside (O-glucoside of ampelopsin A)
 | This article about an aromatic compound is a stub. You can help Wikipedia by expanding it. |
- v
- t
- e










