FNTA
| FNTA | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Identifiers | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Aliases | FNTA, FPTA, PGGT1A, PTAR2, farnesyltransferase, CAAX box, alpha | ||||||||||||||||||||||||||||||||||||||||||||||||||
| External IDs | OMIM: 134635; MGI: 104683; HomoloGene: 1534; GeneCards: FNTA; OMA:FNTA - orthologs | ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
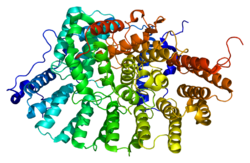
Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha is an enzyme that in humans is encoded by the FNTA gene.[5][6]
Prenyltransferases attach either a farnesyl group or a geranylgeranyl group in thioether linkage to the cysteine residue of protein's with a C-terminal CAAX box. CAAX geranylgeranyltransferase and CAAX farnesyltransferase are heterodimers that share the same alpha subunit but have different beta subunits. This gene encodes the alpha subunit of these transferases. Alternative splicing results in multiple transcript variants encoding different isoforms.[6]
Interactions
FNTA has been shown to interact with TGF beta receptor 1.[7]
References
- ^ a b c GRCh38: Ensembl release 89: ENSG00000168522 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000015994 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ Andres DA, Milatovich A, Ozcelik T, Wenzlau JM, Brown MS, Goldstein JL, Francke U (February 1994). "cDNA cloning of the two subunits of human CAAX farnesyltransferase and chromosomal mapping of FNTA and FNTB loci and related sequences". Genomics. 18 (1): 105–12. doi:10.1006/geno.1993.1432. PMID 8276393.
- ^ a b "Entrez Gene: FNTA farnesyltransferase, CAAX box, alpha".
- ^ Kawabata M, Imamura T, Miyazono K, Engel M E, Moses H L (December 1995). "Interaction of the transforming growth factor-beta type I receptor with farnesyl-protein transferase-alpha". J. Biol. Chem. 270 (50). UNITED STATES: 29628–31. doi:10.1074/jbc.270.50.29628. ISSN 0021-9258. PMID 8530343.
Further reading
- Adamson P, Marshall CJ, Hall A, Tilbrook PA (1992). "Post-translational modifications of p21rho proteins". J. Biol. Chem. 267 (28): 20033–8. doi:10.1016/S0021-9258(19)88661-1. PMID 1400319.
- Manne V, Roberts D, Tobin A, et al. (1990). "Identification and preliminary characterization of protein-cysteine farnesyltransferase". Proc. Natl. Acad. Sci. U.S.A. 87 (19): 7541–5. Bibcode:1990PNAS...87.7541M. doi:10.1073/pnas.87.19.7541. PMC 54783. PMID 2217184.
- Armstrong SA, Hannah VC, Goldstein JL, Brown MS (1995). "CAAX geranylgeranyl transferase transfers farnesyl as efficiently as geranylgeranyl to RhoB". J. Biol. Chem. 270 (14): 7864–8. doi:10.1074/jbc.270.14.7864. PMID 7713879.
- Zhang FL, Diehl RE, Kohl NE, et al. (1994). "cDNA cloning and expression of rat and human protein geranylgeranyltransferase type-I". J. Biol. Chem. 269 (5): 3175–80. doi:10.1016/S0021-9258(17)41845-X. PMID 8106351.
- Sinensky M, Fantle K, Trujillo M, et al. (1994). "The processing pathway of prelamin A." J. Cell Sci. 107. ( Pt 1): 61–7. doi:10.1242/jcs.107.1.61. PMID 8175923.
- Andres DA, Goldstein JL, Ho YK, Brown MS (1993). "Mutational analysis of alpha-subunit of protein farnesyltransferase. Evidence for a catalytic role". J. Biol. Chem. 268 (2): 1383–90. doi:10.1016/S0021-9258(18)54087-4. PMID 8419339.
- Omer CA, Kral AM, Diehl RE, et al. (1993). "Characterization of recombinant human farnesyl-protein transferase: cloning, expression, farnesyl diphosphate binding, and functional homology with yeast prenyl-protein transferases". Biochemistry. 32 (19): 5167–76. doi:10.1021/bi00070a028. PMID 8494894.
- Kawabata M, Imamura T, Miyazono K, et al. (1996). "Interaction of the transforming growth factor-beta type I receptor with farnesyl-protein transferase-alpha". J. Biol. Chem. 270 (50): 29628–31. doi:10.1074/jbc.270.50.29628. PMID 8530343.
- Wang T, Danielson PD, Li BY, et al. (1996). "The p21(RAS) farnesyltransferase alpha subunit in TGF-beta and activin signaling". Science. 271 (5252): 1120–2. Bibcode:1996Sci...271.1120W. doi:10.1126/science.271.5252.1120. PMID 8599089. S2CID 83164753.
- Nantais DE, Schwemmle M, Stickney JT, et al. (1996). "Prenylation of an interferon-gamma-induced GTP-binding protein: the human guanylate binding protein, huGBP1". J. Leukoc. Biol. 60 (3): 423–31. doi:10.1002/jlb.60.3.423. PMID 8830800. S2CID 33727864.
- Bonaldo MF, Lennon G, Soares MB (1997). "Normalization and subtraction: two approaches to facilitate gene discovery". Genome Res. 6 (9): 791–806. doi:10.1101/gr.6.9.791. PMID 8889548.
- Goalstone ML, Draznin B (1996). "Effect of insulin on farnesyltransferase activity in 3T3-L1 adipocytes". J. Biol. Chem. 271 (44): 27585–9. doi:10.1074/jbc.271.44.27585. PMID 8910345.
- Prakash B, Praefcke GJ, Renault L, et al. (2000). "Structure of human guanylate-binding protein 1 representing a unique class of GTP-binding proteins". Nature. 403 (6769): 567–71. Bibcode:2000Natur.403..567P. doi:10.1038/35000617. PMID 10676968. S2CID 4431592.
- Zeng Q, Si X, Horstmann H, et al. (2000). "Prenylation-dependent association of protein-tyrosine phosphatases PRL-1, -2, and -3 with the plasma membrane and the early endosome". J. Biol. Chem. 275 (28): 21444–52. doi:10.1074/jbc.M000453200. PMID 10747914.
- Ashar HR, James L, Gray K, et al. (2000). "Farnesyl transferase inhibitors block the farnesylation of CENP-E and CENP-F and alter the association of CENP-E with the microtubules". J. Biol. Chem. 275 (39): 30451–7. doi:10.1074/jbc.M003469200. PMID 10852915.
- Guenzi E, Töpolt K, Cornali E, et al. (2001). "The helical domain of GBP-1 mediates the inhibition of endothelial cell proliferation by inflammatory cytokines". EMBO J. 20 (20): 5568–77. doi:10.1093/emboj/20.20.5568. PMC 125279. PMID 11598000.
- Long SB, Hancock PJ, Kral AM, et al. (2001). "The crystal structure of human protein farnesyltransferase reveals the basis for inhibition by CaaX tetrapeptides and their mimetics". Proc. Natl. Acad. Sci. U.S.A. 98 (23): 12948–53. Bibcode:2001PNAS...9812948L. doi:10.1073/pnas.241407898. PMC 60805. PMID 11687658.
- Bell IM, Gallicchio SN, Abrams M, et al. (2002). "3-Aminopyrrolidinone farnesyltransferase inhibitors: design of macrocyclic compounds with improved pharmacokinetics and excellent cell potency". J. Med. Chem. 45 (12): 2388–409. doi:10.1021/jm010531d. PMID 12036349.
- Long SB, Casey PJ, Beese LS (2002). "Reaction path of protein farnesyltransferase at atomic resolution". Nature. 419 (6907): 645–50. Bibcode:2002Natur.419..645L. doi:10.1038/nature00986. PMID 12374986. S2CID 4412580.
External links
- Overview of all the structural information available in the PDB for UniProt: P49354 (Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha) at the PDBe-KB.
- v
- t
- e
-
 1d8d: CO-CRYSTAL STRUCTURE OF RAT PROTEIN FARNESYLTRANSFERASE COMPLEXED WITH A K-RAS4B PEPTIDE SUBSTRATE AND FPP ANALOG AT 2.0A RESOLUTION
1d8d: CO-CRYSTAL STRUCTURE OF RAT PROTEIN FARNESYLTRANSFERASE COMPLEXED WITH A K-RAS4B PEPTIDE SUBSTRATE AND FPP ANALOG AT 2.0A RESOLUTION -
 1d8e: Zinc-depleted FTase complexed with K-RAS4B peptide substrate and FPP analog.
1d8e: Zinc-depleted FTase complexed with K-RAS4B peptide substrate and FPP analog. -
 1fpp: PROTEIN FARNESYLTRANSFERASE COMPLEX WITH FARNESYL DIPHOSPHATE
1fpp: PROTEIN FARNESYLTRANSFERASE COMPLEX WITH FARNESYL DIPHOSPHATE -
 1ft1: CRYSTAL STRUCTURE OF PROTEIN FARNESYLTRANSFERASE AT 2.25 ANGSTROMS RESOLUTION
1ft1: CRYSTAL STRUCTURE OF PROTEIN FARNESYLTRANSFERASE AT 2.25 ANGSTROMS RESOLUTION -
 1ft2: CO-CRYSTAL STRUCTURE OF PROTEIN FARNESYLTRANSFERASE COMPLEXED WITH A FARNESYL DIPHOSPHATE SUBSTRATE
1ft2: CO-CRYSTAL STRUCTURE OF PROTEIN FARNESYLTRANSFERASE COMPLEXED WITH A FARNESYL DIPHOSPHATE SUBSTRATE -
 1jcq: CRYSTAL STRUCTURE OF HUMAN PROTEIN FARNESYLTRANSFERASE COMPLEXED WITH FARNESYL DIPHOSPHATE AND THE PEPTIDOMIMETIC INHIBITOR L-739,750
1jcq: CRYSTAL STRUCTURE OF HUMAN PROTEIN FARNESYLTRANSFERASE COMPLEXED WITH FARNESYL DIPHOSPHATE AND THE PEPTIDOMIMETIC INHIBITOR L-739,750 -
 1jcr: CRYSTAL STRUCTURE OF RAT PROTEIN FARNESYLTRANSFERASE COMPLEXED WITH THE NON-SUBSTRATE TETRAPEPTIDE INHIBITOR CVFM AND FARNESYL DIPHOSPHATE SUBSTRATE
1jcr: CRYSTAL STRUCTURE OF RAT PROTEIN FARNESYLTRANSFERASE COMPLEXED WITH THE NON-SUBSTRATE TETRAPEPTIDE INHIBITOR CVFM AND FARNESYL DIPHOSPHATE SUBSTRATE -
 1jcs: CRYSTAL STRUCTURE OF RAT PROTEIN FARNESYLTRANSFERASE COMPLEXED WITH THE PEPTIDE SUBSTRATE TKCVFM AND AN ANALOG OF FARNESYL DIPHOSPHATE
1jcs: CRYSTAL STRUCTURE OF RAT PROTEIN FARNESYLTRANSFERASE COMPLEXED WITH THE PEPTIDE SUBSTRATE TKCVFM AND AN ANALOG OF FARNESYL DIPHOSPHATE -
 1kzo: PROTEIN FARNESYLTRANSFERASE COMPLEXED WITH FARNESYLATED K-RAS4B PEPTIDE PRODUCT AND FARNESYL DIPHOSPHATE SUBSTRATE BOUND SIMULTANEOUSLY
1kzo: PROTEIN FARNESYLTRANSFERASE COMPLEXED WITH FARNESYLATED K-RAS4B PEPTIDE PRODUCT AND FARNESYL DIPHOSPHATE SUBSTRATE BOUND SIMULTANEOUSLY -
 1kzp: PROTEIN FARNESYLTRANSFERASE COMPLEXED WITH A FARNESYLATED K-RAS4B PEPTIDE PRODUCT
1kzp: PROTEIN FARNESYLTRANSFERASE COMPLEXED WITH A FARNESYLATED K-RAS4B PEPTIDE PRODUCT -
 1ld7: Co-crystal structure of Human Farnesyltransferase with farnesyldiphosphate and inhibitor compound 66
1ld7: Co-crystal structure of Human Farnesyltransferase with farnesyldiphosphate and inhibitor compound 66 -
 1ld8: Co-crystal structure of Human Farnesyltransferase with farnesyldiphosphate and inhibitor compound 49
1ld8: Co-crystal structure of Human Farnesyltransferase with farnesyldiphosphate and inhibitor compound 49 -
 1mzc: Co-Crystal Structure Of Human Farnesyltransferase With Farnesyldiphosphate and Inhibitor Compound 33a
1mzc: Co-Crystal Structure Of Human Farnesyltransferase With Farnesyldiphosphate and Inhibitor Compound 33a -
 1n4p: Protein Geranylgeranyltransferase type-I Complexed with Geranylgeranyl Diphosphate
1n4p: Protein Geranylgeranyltransferase type-I Complexed with Geranylgeranyl Diphosphate -
 1n4q: Protein Geranylgeranyltransferase type-I Complexed with a GGPP Analog and a KKKSKTKCVIL Peptide
1n4q: Protein Geranylgeranyltransferase type-I Complexed with a GGPP Analog and a KKKSKTKCVIL Peptide -
 1n4r: Protein Geranylgeranyltransferase type-I Complexed with a Geranylgeranylated KKKSKTKCVIL Peptide Product
1n4r: Protein Geranylgeranyltransferase type-I Complexed with a Geranylgeranylated KKKSKTKCVIL Peptide Product -
 1n4s: Protein Geranylgeranyltransferase type-I Complexed with GGPP and a Geranylgeranylated KKKSKTKCVIL Peptide Product
1n4s: Protein Geranylgeranyltransferase type-I Complexed with GGPP and a Geranylgeranylated KKKSKTKCVIL Peptide Product -
 1n94: Aryl Tetrahydropyridine Inhbitors of Farnesyltransferase: Glycine, Phenylalanine and Histidine Derivates
1n94: Aryl Tetrahydropyridine Inhbitors of Farnesyltransferase: Glycine, Phenylalanine and Histidine Derivates -
 1n95: Aryl Tetrahydrophyridine Inhbitors of Farnesyltranferase: Glycine, Phenylalanine and Histidine Derivatives
1n95: Aryl Tetrahydrophyridine Inhbitors of Farnesyltranferase: Glycine, Phenylalanine and Histidine Derivatives -
 1n9a: Farnesyltransferase complex with tetrahydropyridine inhibitors
1n9a: Farnesyltransferase complex with tetrahydropyridine inhibitors -
 1ni1: Imidazole and cyanophenyl farnesyl transferase inhibitors
1ni1: Imidazole and cyanophenyl farnesyl transferase inhibitors -
 1nl4: Crystal Structure of Rat Farnesyl Transferase in Complex With A Potent Biphenyl Inhibitor
1nl4: Crystal Structure of Rat Farnesyl Transferase in Complex With A Potent Biphenyl Inhibitor -
 1o1r: Structure of FPT bound to GGPP
1o1r: Structure of FPT bound to GGPP -
 1o1s: Structure of FPT bound to isoprenoid analog 3b
1o1s: Structure of FPT bound to isoprenoid analog 3b -
 1o1t: Structure of FPT bound to the CVIM-FPP product
1o1t: Structure of FPT bound to the CVIM-FPP product -
 1o5m: Structure of FPT bound to the inhibitor SCH66336
1o5m: Structure of FPT bound to the inhibitor SCH66336 -
 1qbq: STRUCTURE OF RAT FARNESYL PROTEIN TRANSFERASE COMPLEXED WITH A CVIM PEPTIDE AND ALPHA-HYDROXYFARNESYLPHOSPHONIC ACID.
1qbq: STRUCTURE OF RAT FARNESYL PROTEIN TRANSFERASE COMPLEXED WITH A CVIM PEPTIDE AND ALPHA-HYDROXYFARNESYLPHOSPHONIC ACID. -
 1s63: Human protein farnesyltransferase complexed with L-778,123 and FPP
1s63: Human protein farnesyltransferase complexed with L-778,123 and FPP -
 1s64: Rat protein geranylgeranyltransferase type-I complexed with L-778,123 and a sulfate anion
1s64: Rat protein geranylgeranyltransferase type-I complexed with L-778,123 and a sulfate anion -
 1sa4: human protein farnesyltransferase complexed with FPP and R115777
1sa4: human protein farnesyltransferase complexed with FPP and R115777 -
 1sa5: Rat protein farnesyltransferase complexed with FPP and BMS-214662
1sa5: Rat protein farnesyltransferase complexed with FPP and BMS-214662 -
 1tn6: Protein Farnesyltransferase Complexed with a Rap2a Peptide Substrate and a FPP Analog at 1.8A Resolution
1tn6: Protein Farnesyltransferase Complexed with a Rap2a Peptide Substrate and a FPP Analog at 1.8A Resolution -
 1tn7: Protein Farnesyltransferase Complexed with a TC21 Peptide Substrate and a FPP Analog at 2.3A Resolution
1tn7: Protein Farnesyltransferase Complexed with a TC21 Peptide Substrate and a FPP Analog at 2.3A Resolution -
 1tn8: Protein Farnesyltransferase Complexed with a H-Ras Peptide Substrate and a FPP Analog at 2.25A Resolution
1tn8: Protein Farnesyltransferase Complexed with a H-Ras Peptide Substrate and a FPP Analog at 2.25A Resolution -
 1tnb: Rat Protein Geranylgeranyltransferase Type-I Complexed with a GGPP analog and a substrate KKSKTKCVIF Peptide Derived from TC21
1tnb: Rat Protein Geranylgeranyltransferase Type-I Complexed with a GGPP analog and a substrate KKSKTKCVIF Peptide Derived from TC21 -
 1tno: Rat Protein Geranylgeranyltransferase Type-I Complexed with a GGPP analog and a KKKSKTKCVIM Peptide Derived from K-Ras4B
1tno: Rat Protein Geranylgeranyltransferase Type-I Complexed with a GGPP analog and a KKKSKTKCVIM Peptide Derived from K-Ras4B -
 1tnu: Rat Protein Geranylgeranyltransferase Type-I Complexed with a GGPP analog and a GCINCCKVL Peptide Derived from RhoB
1tnu: Rat Protein Geranylgeranyltransferase Type-I Complexed with a GGPP analog and a GCINCCKVL Peptide Derived from RhoB -
 1tny: Rat Protein Geranylgeranyltransferase Type-I Complexed with a GGPP analog and a FREKKFFCAIL Peptide Derived from the Heterotrimeric G Protein Gamma-2 Subunit
1tny: Rat Protein Geranylgeranyltransferase Type-I Complexed with a GGPP analog and a FREKKFFCAIL Peptide Derived from the Heterotrimeric G Protein Gamma-2 Subunit -
 1tnz: Rat Protein Geranylgeranyltransferase Type-I Complexed with a GGPP analog and a RRCVLL Peptide Derived from Cdc42 splice isoform-2
1tnz: Rat Protein Geranylgeranyltransferase Type-I Complexed with a GGPP analog and a RRCVLL Peptide Derived from Cdc42 splice isoform-2 -
 1x81: Farnesyl transferase structure of Jansen compound
1x81: Farnesyl transferase structure of Jansen compound -
 2bed: Structure of FPT bound to inhibitor SCH207736
2bed: Structure of FPT bound to inhibitor SCH207736 -
 2f0y: Crystal Structure Of Human Protein Farnesyltransferase Complexed With Farnesyl Diphosphate and hydantoin derivative
2f0y: Crystal Structure Of Human Protein Farnesyltransferase Complexed With Farnesyl Diphosphate and hydantoin derivative -
 2h6f: Protein Farnesyltransferase Complexed with a Farnesylated DDPTASACVLS Peptide Product at 1.5A Resolution
2h6f: Protein Farnesyltransferase Complexed with a Farnesylated DDPTASACVLS Peptide Product at 1.5A Resolution -
 2h6g: W102T Protein Farnesyltransferase Mutant Complexed with a Geranylgeranylated DDPTASACVLS Peptide Product at 1.85A Resolution
2h6g: W102T Protein Farnesyltransferase Mutant Complexed with a Geranylgeranylated DDPTASACVLS Peptide Product at 1.85A Resolution -
 2h6h: Y365F Protein Farnesyltransferase Mutant Complexed with a Farnesylated DDPTASACVLS Peptide Product at 1.8A
2h6h: Y365F Protein Farnesyltransferase Mutant Complexed with a Farnesylated DDPTASACVLS Peptide Product at 1.8A -
 2h6i: W102T/Y365F Protein Farnesyltransferase Double Mutant Complexed with a Geranylgeranylated DDPTASACVLS Peptide Product at 3.0A
2h6i: W102T/Y365F Protein Farnesyltransferase Double Mutant Complexed with a Geranylgeranylated DDPTASACVLS Peptide Product at 3.0A -
 2iej: Human Protein Farnesyltransferase Complexed with Inhibitor Compound STN-48 And FPP Analog at 1.8A Resolution
2iej: Human Protein Farnesyltransferase Complexed with Inhibitor Compound STN-48 And FPP Analog at 1.8A Resolution
 | This article on a gene on human chromosome 8 is a stub. You can help Wikipedia by expanding it. |
- v
- t
- e

































































